Neon
During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very common element in the universe and solar system (it is fifth in cosmic abundance after hydrogen, helium, oxygen and carbon), it is very rare on Earth. It composes about 18.2 ppm of air by volume (this is about the same as the molecular or mole fraction), and a smaller fraction in the crust. The reason for neon's relative scarcity on Earth and the inner (terrestrial) planets, is that neon forms no compounds to fix it to solids, and is highly volatile, therefore escaping from the planetesimals under the warmth of the newly-ignited Sun in the early Solar System. Even the atmosphere of Jupiter is somewhat depleted of neon, presumably for this reason.
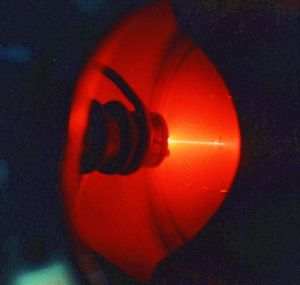
Neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or in high-voltage discharge tubes or neon advertising signs. The red emission line from neon is also responsible for the well known red light of helium-neon lasers. Neon is used in a few plasma tube and refrigerant applications but has few other commercial uses. It is commercially extracted by the fractional distillation of liquid air. It is considerably more expensive than helium, since air is its only source.
Occurrence
Stable isotopes of neon are produced in stars. 20Ne is created in fusing helium and oxygen in the alpha process, which requires temperatures above 100 megakelvins and masses greater than 3 solar masses.
Neon is abundant on a universal scale; it is the fifth most abundant chemical element in the universe by mass, after hydrogen, helium, oxygen, and carbon (see chemical element). Its relative rarity on Earth, like that of helium, is due to its relative lightness, high vapor pressure at very low temperatures, and chemical inertness, all properties which tend to keep it from being trapped in the condensing gas and dust clouds which resulted in the formation of smaller and warmer solid planets like Earth.
Neon is monatomic, making it lighter than the molecules of diatomic nitrogen and oxygen which form the bulk of Earth's atmosphere; a balloon filled with neon will rise in air, albeit more slowly than a helium balloon.
Mass abundance in the universe is about 1 part in 750 and in the Sun and presumably in the proto-solar system nebula, about 1 part in 600. The Galileo spacecraft atmospheric entry probe found that even in the upper atmosphere of Jupiter, the abundance of neon is reduced (depleted) by about a factor of 10, to a level of 1 part in 6,000 by mass. This may indicate that even the ice-planetesimals which brought neon into Jupiter from the outer solar system, formed in a region which was too warm for them to have kept their neon (abundances of heavier inert gases on Jupiter are several times that found in the Sun).
Neon is rare on Earth, found in the Earth's atmosphere at 1 part in 55,000, or 18.2 ppm by volume (this is about the same as the molecule or mole fraction), or 1 part in 79,000 of air by mass. It comprises a smaller fraction in the crust. It is industrially produced by cryogenic fractional distillation of liquefied air.
| Symbol | Ne | |
| Atomic Number | 10 | |
| Atomic Weight | 20.1797 | |
| Oxidation States | 0 | |
| Electronegativity, Pauling | ||
| State at RT | Gas, Nonmetal | |
| Melting Point, K | 24,48 | |
| Boiling Point, K | 27.1 |
Interesting Facts about Neon
- 0.0018 percent of Earth’s atmosphere is neon.
- Although it is relatively rare on our planet, neon is the fifth most abundant element in the universe.
- If you could gather all the neon from the rooms in a typical new home in the United States, you would get 10 liters (2 gallons) of neon gas.
- Neon forms in stars with a mass of eight or more Earth suns.
- Near the end of their lives, these stars enter the carbon burning phase, also making oxygen, sodium and magnesium. (For oxygen production, stars need a mass of ‘just’ five of our suns.)
- Neon has no stable compounds.
Appearance and Characteristics
Harmful effects:
Neon is not known to be toxic.
Characteristics:
- Neon is a light, very inert gas.
- Colorless under normal conditions, it glows a reddish-orange in a vacuum discharge tube.
- Neon forms no known stable compounds.
- It has the smallest liquid range of any element (2.6 oC).
Uses of Neon
- When a few thousand volts are applied to neon, it emits an orange/red light. It is therefore often used in brightly lit advertising signs. Georges Claude was the first person to make glass tubes of neon in 1910. He later bent the glass tubes to makes letters that glowed and produced the first neon advertising signs.
- Neon is also used in high-voltage warning indicators, in Geiger counters and in television tubes.
- Liquid neon is used as a cryogenic refrigerant.